Answer:
Boron and Aluminum
Step-by-step explanation:
If you write the electron configuration for boron and aluminum, you get:
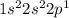 for boron and
for boron and
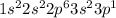 for aluminum. Both have 3 valance electrons and has 2 electrons in a s-orbital and 1 in a p-orbital. These valance electron similarities are based on the column/group the elements are. Therefore, Boron and Aluminum have similar chemical behaviours and similar arrangement of outer/valance electrons.
for aluminum. Both have 3 valance electrons and has 2 electrons in a s-orbital and 1 in a p-orbital. These valance electron similarities are based on the column/group the elements are. Therefore, Boron and Aluminum have similar chemical behaviours and similar arrangement of outer/valance electrons.