Answer:
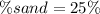
Step-by-step explanation:
Hello.
In this case, given the mass of the mixture, we can define it in terms of the mass of sand and table salt as shown below:
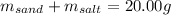
Moreover, as after filtering, the mass of dry sand turns out 5.00 g, we can compute the % sand in the original mixture by dividing this value over the mass of the mixture as shown below:
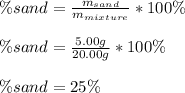
Best regards!