Answer:
Approximately
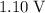 under standard conditions.
under standard conditions.
Step-by-step explanation:
Equation for the overall reaction:
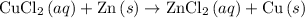 .
.
Write down the ionic equation for this reaction:
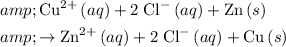 .
.
The net ionic equation for this reaction would be:
 .
.
In this reaction:
- Zinc loses electrons and was oxidized (at the anode):
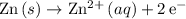 .
. - Copper gains electrons and was reduced (at the cathode):
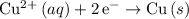 .
.
Look up the standard potentials for each half-reaction on a table of standard reduction potentials.
Notice that
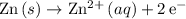 is oxidation and is likely not on the table of standard reduction potentials. However, the reverse reaction,
is oxidation and is likely not on the table of standard reduction potentials. However, the reverse reaction,
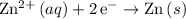 , is reduction and is likely on the table.
, is reduction and is likely on the table.
The reduction potential of
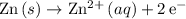 would be
would be
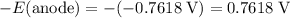 , the opposite of the reverse reaction
, the opposite of the reverse reaction
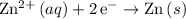 .
.
The standard potential of the overall reaction would be the sum of the standard potentials of the two half-reactions:
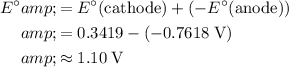 .
.