Answer:
The answer is
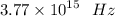
Step-by-step explanation:
The frequency of the photon can be found by using the formula
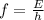
where
E is the energy
f is the frequency
h is the Planck's constant which is
6.626 × 10-³⁴ Js
From the question
E = 2.50 × 10^-18 J
So we have
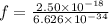
We have the final answer as
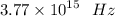
Hope this helps you