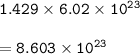Number of particles : 8.603 x 10²³
Further explanation
Standard conditions for temperature and pressure are used as a reference in certain calculations or conditions
Assumption⇒ Standard Conditions
Conditions at T 0 ° C and P 1 atm are stated by STP (Standard Temperature and Pressure). At STP, Vm is 22.4 liters/mol.
1 mol = 6.02 x 10²³ particles (atoms, ions, or molecules)
32.0 liters of Helium
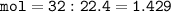
Number of particles :