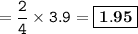Moles of Fe₂O₃ formed : 1.95
Further explanation
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
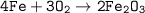
We find the limiting reactant from the ratio of the mole and the reaction coefficient
Fe ⇒ 3.9 : 4 = 0.975
O ⇒ 4.7 : 3 = 1.56
Because the ratio of Fe is smaller, then Fe becomes the limiting reactant
Reaction
4Fe + 3O₂ ⇒ 2Fe₂O₃
mol Fe₂O₃ from mol of Fe as limiting reactant
mol ratio Fe₂O₃ : Fe = 2 : 4, then mol Fe₂O₃ :