Answer:
Follows are the solution:
Step-by-step explanation:
A + B = C
Its response decreases over time as well as consumption of a reactants.
r = -kAB
during response A convert into 2x while B convert into x to form 3x of C
let's y = C
y = 3x
Still not converted sum of reaction
for A: 100 - 2x
for B: 50 - x
Shift of x over time
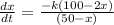
Integration of x as regards t
![(1)/([(100 - 2x)(50 - x)]) dx = -k dt\\\\(1)/(2[(50 - x)(50 - x)]) dx = -k dt\\\\\ integral\ (1)/(2[(50 - x)^2]) dx =\ integral [-k ] \ dt\\\\(-1)/([100-2x]) = -kt + D \\\\](https://img.qammunity.org/2021/formulas/chemistry/high-school/ftr2uclda66bmfwb8jah72419lfvwdzazw.png)
D is the constant of integration
initial conditions: t = 0, x = 0
![(-1)/([100-2x]) = -kt + D \\\\( -1)/([100]) = 0 + D\\\\D= (-1)/(100)\\\\](https://img.qammunity.org/2021/formulas/chemistry/high-school/mqca55b2d1kds2x5r4m0rl4d5b30lem0da.png)
hence we get:
![(-1)/([100-2x])= -kt -(1)/(100)\\\\or \\\\ (1)/((100-2x)) = kt + (1)/(100)](https://img.qammunity.org/2021/formulas/chemistry/high-school/ma09kxr2q8oo0pbtdbgw9cp4relwe2xy7r.png)
after t = 7 minutes ,
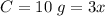
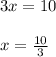
Insert the above value x into
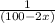 equation
equation
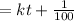 to get k.
to get k.
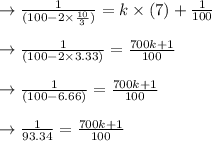

therefore plugging in the equation the above value of k
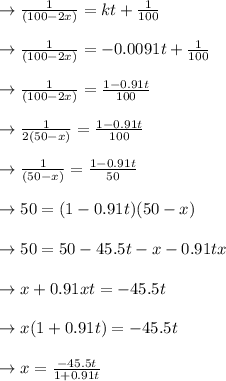
Let y = C
, calculate C:
y = 3x
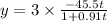
amount of C formed in 28 mins
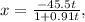 plug t = 28
plug t = 28
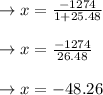
therefore amount of C formed in 28 minutes is = 3x = 144.78 grams
C:
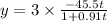
y= 136.5 =137