Answer:
The absolute uncertainty is approximately 1.69 × 10⁻³
Step-by-step explanation:
The volume needed for NaOH needed to make the solution = 16.66 ml
The wt% of the added NaOH = 53.4 wt%
The volume of the NaOH to be prepared = 2.00 L
The concentration of the NaOH to be prepared = 0.169 M
The molar mass of NaOH = 39.997 g/mol
Therefore, 100 g of sample contains 53.4 g of NaOH
The mass of the sample = 16.66 × 1.52 = 25.3232 g
The mass of NaOH in the sample = 0.534 × 25.3232 = 13.5225888 g ≈ 13.52 g
Therefore;
The number of moles of NaOH = 13.52/39.9971 = 0.3381 moles
Therefore, we have 0.3381 moles in 2.00L solution, which gives;
The number of moles per liter = 0.3881/2 = 0.169045 moles/liter
The molarity ≈ 0.169 M
The absolute uncertainty, u(c) is given as follows;
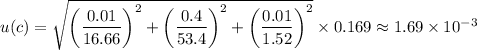
The absolute uncertainty, u(c) ≈ 1.69 × 10⁻³.