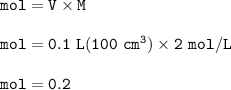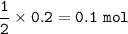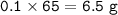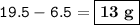The mass of unreacted zinc = 13 g
Further explanation
The reaction equation is the chemical formula of reactants and products
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reactants and products
Reaction
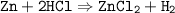
mol ratio Zn : HCl = 1 : 2