The balance of chemical equation : 2KClO₃ ⟶ 2KCl + 3O₂
Further explanation
Equalization (balance) of chemical reactions can be done using variables. Steps in equalizing the reaction equation:
- 1. gives a coefficient on substances involved in the equation of reaction such as a, b, or c, etc.
- 2. make an equation based on the similarity of the number of atoms where the number of atoms = coefficient × index (subscript) between reactant and product
- 3. Select the coefficient of the substance with the most complex chemical formula equal to 1
Reaction
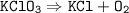
1. Coefficient
KClO₃ ⟶ aKCl + bO₂
2. Equation
K ⇒ left =1, right=a ⇒a=1
Cl⇒ left =1, right=a ⇒a=1
O ⇒ left = 3, right = 2b ⇒3=2b, b = 1.5
The equation turns into :
KClO₃ ⟶ KCl + 1.5O₂
multiplied by 2
2KClO₃ ⟶ 2KCl + 3O₂