Answer:
Gold
Silver
Mixture
Step-by-step explanation:
Density
The density of an object of mass m and volume V is given by:
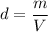
We are given the following densities:
Gold: 19.3 gr/cm3
Silver: 10.5 gr/cm3
With the data provided in the table, we can confirm which of the three columns corresponds to gold, silver, or a mix of both.
Calculating the first density: m1=1,930 gr, V1=100 cm3
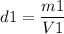
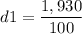
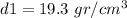
This crown is made of gold
Calculating the second density: m2=1,930 gr, V2=184 cm3
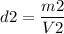
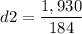
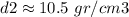
This crown is made of silver
Calculating the last density: m3=1,930 gr, V3=150 cm3
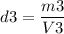
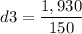
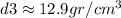
Since the value of the density is between gold and silver, this crown is a mixture.