Answer:
Option (2)
Explanation:
pH level of a substance is determined by the formula,
pH = - log[H+}
where [H+] is the hydrogen ion concentration
Let the [H+] ion concentration = a
a = -log[H+]
log[H+] = -a
[H+] = 1 ×
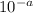
Here 'a' is between 7 and 14.
Which means an alkaline substance must have the concentration between
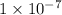 to
to
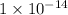 .
.
From the given options,
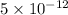 moles per liter lies in the given range.
moles per liter lies in the given range.
Therefore, Option (2) will be the answer.