Answer:
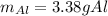
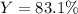
Step-by-step explanation:
Hello.
In this case, for the given balanced reaction:
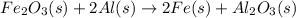
For 10.0 g of iron (III) oxide (molar mass = 160 g/mol), based on the 1:2 mole ratio with Al (atomic mass = 27 g/mol), the required mass is then:
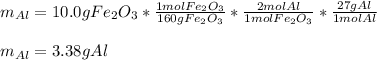
Moreover, as 5.3 g of aluminum oxide are actually yielded, from the 10.0 g of iron (III) oxide, we can compute the theoretical mass of aluminum oxide (molar mass = 102 g/mol) via their 1:1 mole ratio:
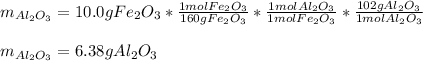
Thus, the percent yield (actual/theoretical*100%) turns out:
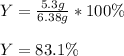
Best regards.