Answer:
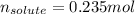
Step-by-step explanation:
Hello.
In this case, we are talking about molarity which is an unit of concentration relating the moles of the solute and the volume of the solution in liters only:
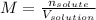
Since the solute is the (NH4)2Fe(SO4)2 and the volume in liters:
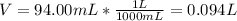
Thus, for the given 2.50-M solution, the moles of solute result:
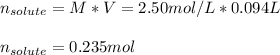
Best regards.