Step-by-step explanation:
In chemistry, a mole is the base unit of the amount of substance in a system containing elementary entities (atoms, molecules, ions, electrons), and one mole of substance corresponds to the Avogadro's constant or
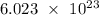 entities. The stoichiometry equation that relates the amount of substance into the measurement in moles is
entities. The stoichiometry equation that relates the amount of substance into the measurement in moles is
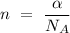 ,
,
where
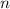 is the number of moles,
is the number of moles,
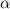 is the amount of substance and
is the amount of substance and
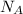 is the Avogadro's constant.
is the Avogadro's constant.
Therefore,
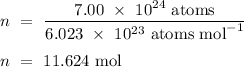 .
.