Answer:
42 pounds per square inches.
Explanation:
The temperature stays the same, the volume of a gas is inversely proportional to the pressure of the gas.
⇒
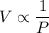
or
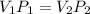
Volume, V₁ = 183 cubic inches
Pressure, P₁ = 14 pounds/square inch
We need to find the new pressure if the volume is decreased to 61 cubic inches. Let P₂ is new pressure. So,

Hence, the final pressure is 42 pounds per square inches.