Answer:
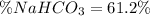
Step-by-step explanation:
Hello.
In this case, since the reaction between sodium bicarbonate and a symbolic HA is:
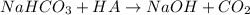
For 0.561 g of carbon dioxide (molar mass = 44 g/mol) we can compute the required mass of sodium bicarbonate (molar mass = 84 g/mol ) via stoichiometry:
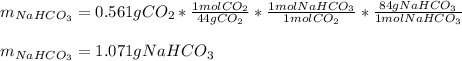
Then, the percent by mass of sodium bicarbonate in the original mixture is obtained by dividing the mass of the sodium bicarbonate from which the CO2 was yielded by the total mass of the mixture:
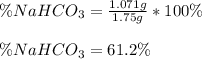
Best regards.