Answer:
O2, oxygen.
Step-by-step explanation:
Hello.
In this case, for the undergoing chemical reaction, we need to compute the moles of CO2 yielded by 85 g of CH4 (molar mass = 16 g/mol) and by 320 g of O2 (molar mass 32 g/mol) via the following mole-mass relationships:
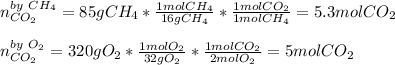
Considering the 1:2:1 among CH4, O2 and CO2. Therefore, since 320 g of O2 yield the smallest amount of CO2 we infer that the limiting reactant is O2.
Best regards.