Answer:
5496 g of epinephrine
Step-by-step explanation:
From the question, 5.00 moles of epinephrine is required to make Epipen.
Molar Mass of epinephrine = 183.20 g/mol
mass of 5 moles of epinephrine=
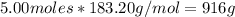
Therefore, 30.00 moles of epinephrine needs 30.00 * 916/5 =5496 g of epinephrine