Answer:
Due to the specific heat capacity of iron, 0.444 J/(g·°C), is more than the specific heat capacity for lead, 0.160 J/(g·°C)
Step-by-step explanation:
The given parameters are;
The metals provided to melt the ice and their temperature includes;
One kg (1000 g) of iron;
Specific heat capacity = 0.444 J/(g·°C)
Temperature = 100°C
1 kg (1000 g) of lead
Specific heat capacity = 0.160 J/(g·°C)
Temperature = 100°C
Therefore, the heat provided to the ice of mass m, and latent heat of 334 J/g at 0°C by the metals are as follows;
For iron, we have;
ΔQ = Mass × Specific heat capacity × Temperature change
ΔQ
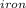 = Heat obtained from the iron by the ice
= Heat obtained from the iron by the ice
ΔQ
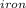 = 0.444 m × 1000 × (100 - 0) = 44400 J
= 0.444 m × 1000 × (100 - 0) = 44400 J
Heat absorbed by the ice for melting, H
 = Heat obtained from the iron
= Heat obtained from the iron
∴ Heat absorbed by the ice for melting, H
 = Mass of ice × Latent heat of ice
= Mass of ice × Latent heat of ice
H
 = Mass of ice × 334 J/g = 44400 J
= Mass of ice × 334 J/g = 44400 J
Mass of ice melted by the iron = 44400 J/334 (J/g) ≈ 132.9 g
Mass of ice melted by the iron ≈ 132.9 g
For lead, we have;
ΔQ = Mass × Specific heat capacity × Temperature change
ΔQ
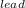 = Heat obtained from the iron by the ice
= Heat obtained from the iron by the ice
ΔQ
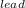 = 0.160 m × 1000 × (100 - 0) = 16000 J
= 0.160 m × 1000 × (100 - 0) = 16000 J
Heat absorbed by the ice for melting, H
 = Heat obtained from the iron
= Heat obtained from the iron
∴ Heat absorbed by the ice for melting, H
 = Mass of ice × Latent heat of ice
= Mass of ice × Latent heat of ice
H
 = Mass of ice × 334 J/g = 16000 J
= Mass of ice × 334 J/g = 16000 J
Mass of ice melted by the lead = 16000 J/334 (J/g) ≈ 47.9 g
Mass of ice melted by the lead ≈ 47.9 g
Therefore, mass of ice melted by the iron, approximately 132.9 g, is more than mass of ice melted by the lead, approximately 47.9 g.