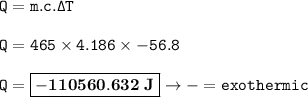The heat released : -110560.632 J
Further explanation
Heat can be calculated using the formula:
Q = mc∆T
Q = heat, J
m = mass, g
c = specific heat, joules / g ° C
∆T = temperature difference, ° C / K
Known
m =465 g
c water = 4.186 J/gram °C
∆T = temperature difference = 18.2 - 75 = - 56.8 °C
Then the heat :