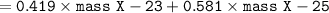Further explanation
Isotopes : the same atomic number, but different mass numbers
Atomic mass is the average atomic mass of all its isotopes
avg atomic mass = m1.%m1 + m2.%m2....mn.%mn
You did not include the mass of each isotope
Element X :
X-23 = 41.9%
X-25 = 58.1%
The average atomic mass of Element X :