Answer:
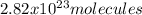
Step-by-step explanation:
Hello.
In this case, given the volume (1cm³=1mL) and density of the bromine we are to firstly compute the mass since it will allow us to compute the representative particles:
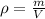
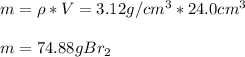
Next, since the mass of one mole of diatomic bromine is 159.82 g (one bromine weights 78.91), we can next compute the moles in that sample:
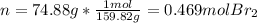
Finally, via the Avogadro's number we can compute the representative particles of bromine as follows:
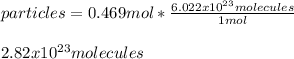
Best regards.