Answer:
a. Combustion.
b.
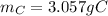
c.
Step-by-step explanation:
Hello.
a. In this case, given the reaction by which propane is converted into carbon dioxide and water:
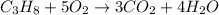
It is known as a combustion reaction since it is about a fuel (here propane) which is burnt by the oxygen contained in the air (21%).
b. Since the carbon dioxide contains all the carbon in the products based on the law of conservation of mass, the yielded grams are computed via a mole mass relationship:
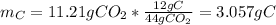
Since 44 grams of carbon dioxide contain 12 grams of carbon.
c. As well as b., all the hydrogen is given off in form of water, thus, the required mass turns out:
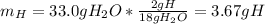
Since 18 grams of water contain 2 grams of hydrogen.
Best regards.