Answer:
Step-by-step explanation:
From the given information:
mass of silver chloride AgCl = 11.89 g
molar mass of AgCl = 143.37 g/mol
We know that:
number of moles = mass/molar mass
∴
number of moles of AgCl = 11.89 g/ 143.37 g/mol
number of moles of AgCl = 0.0829 mol
The chemical equation for the mineral called trona is:
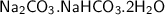
when being reacted with hydrochloric acid, we have:
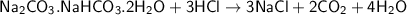
One mole of NaCl formed from one mole of trona sample = 0.0829 moles of AgCl
i.e. 0.0829 moles of NaCl can be formed from AgCl
mass of trona sample = number of moles × molar mass
mass of trona sample = 0.0829 × 226
mass of trona sample = 18.735 g
The mass in the percentage of NaHCO₃ = mass of NaHCO₃/ mass of trona
The mass in the percentage of NaHCO₃ = 6.93/18.735
The mass in the percentage of NaHCO₃ = 0.36989
The mass in the percentage of NaHCO₃ = 36.99%
Nonetheless, a 6.78 g samples manufactured from sodium carbonate in pure 100%
∴
6.78 g sample manufactured from Na₂CO₃ is purer.