Answer : The concentration of the HCl in original bottle is 0.56 M.
Explanation :
According to neutralization law:

where,
 = concentration of HCl
= concentration of HCl
 = concentration of NaOH
= concentration of NaOH
 = volume of HCl
= volume of HCl
 = volume of NaOH
= volume of NaOH
Given:
 = ?
= ?
 = 0.25 M
= 0.25 M
 = 10 mL
= 10 mL
 = 22.4 mL
= 22.4 mL
Now put all the given values in the above formula, we get:
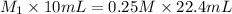
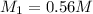
Therefore, the concentration of the HCl in original bottle is 0.56 M.