Given parameters:
Weight of hydrated sample = 20g
Temperature = 250°C
Weight after cooling = 16.5g
Unknown:
Weight of water lost from the sample = ?
Solution:
The weight of water lost from the sample;
Weight of water lost = Weight of hydrated sample - Weight of dry sample
Weight of water lost = 20g - 16.5g = 3.5g
% of water in the sample =
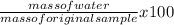
Input parameters solve;
=
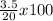
=17.5%