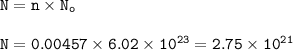The number of particles : 2.75 x 10²¹
Further explanation
A mole is a unit of many particles (atoms, molecules, ions) where 1 mole is the number of particles contained in a substance that is the same amount as many atoms in 12 gr C-12
1 mole = 6.02.10²³ particles
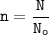
N = number of particles
No = Avogadro number (6.02.10²³)
n = number of moles
While the number of moles can also be obtained by dividing the mass (in grams) with the molar mass of element or molecule
0.75 gram sample of Calcium Nitrate; Ca(NO₃)₂
MW of Ca(NO₃)₂ : 164.09 g/mol
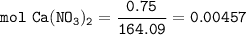
The number of particles