The correct answer is option B. Pauli Exclusion Principle.
The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. This means that no two electrons can have the same principal quantum number (n), the same angular momentum quantum number (l), the same magnetic quantum number (
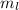 ), and the same spin quantum number (
), and the same spin quantum number (
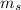 ).
).
The image you provided shows three electrons in the same 2p orbital, all with the same spin. This violates the Pauli Exclusion Principle, because no two electrons can have the same set of four quantum numbers.
Therefore, the answer is B. Pauli Exclusion Principle.