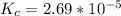Complete question
The complete question is shown on the first uploaded image
Answer:
The value is
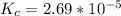
Step-by-step explanation:
From the question we are told that
The equation is
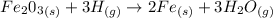
Generally the equilibrium is mathematically represented as
![K_c = ([H_2O]^2)/([H_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/college/oxwwl165xq4wp6cveha7l61ot9ihknlbed.png)
Here
![[H_2O]](https://img.qammunity.org/2021/formulas/chemistry/college/iy5dpjdfwcdykvk2kscx785j1lj1v2anvo.png) is the concentration of water vapor which is mathematically represented as
is the concentration of water vapor which is mathematically represented as
![[H_2O ] = (n_w)/(V_s )](https://img.qammunity.org/2021/formulas/chemistry/college/cupn5javts86g4p44fm9vraddzzpigtee8.png)
Here
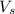 is the volume of the solution given as 8.9 L
is the volume of the solution given as 8.9 L
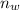 is the number of moles of water vapor which is mathematically represented as
is the number of moles of water vapor which is mathematically represented as
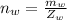
Here
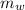 is the mass of water given as 2.00 g
is the mass of water given as 2.00 g
and
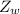 is the molar mass of water with value 18 g/mol
is the molar mass of water with value 18 g/mol
So
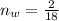
=>
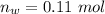
So
![[H_2O ] = (0.11)/(8.9 )](https://img.qammunity.org/2021/formulas/chemistry/college/jcygzj6qwlk5ainkm8k0837ixb5n5blqk3.png)
=>
![[H_2O ] = 0.01236 \ M](https://img.qammunity.org/2021/formulas/chemistry/college/o5hg3jhd9axv8b67ugot2edt6ut41ppu51.png)
Also
![[H]](https://img.qammunity.org/2021/formulas/chemistry/college/vg96h6hx7vmv80qwfi4ds5naer6e33fyno.png) is the concentration of hydrogen gas which is mathematically represented as
is the concentration of hydrogen gas which is mathematically represented as
![[H ] = (n_v)/(V_s )](https://img.qammunity.org/2021/formulas/chemistry/college/vj79br5rrt75vp2yy1ilsquz1qr68qz7de.png)
Here
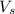 is the volume of the solution given as 8.9 L
is the volume of the solution given as 8.9 L
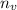 is the number of moles of hydrogen gas which is mathematically represented as
is the number of moles of hydrogen gas which is mathematically represented as
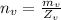
Here
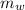 is the mass of water given as 4.77 g
is the mass of water given as 4.77 g
and
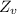 is the molar mass of water with value 2 g/mol
is the molar mass of water with value 2 g/mol
So
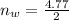
=>
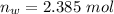
So
![[H_2O ] = (2.385)/(8.9 )](https://img.qammunity.org/2021/formulas/chemistry/college/5a7odvsldvyzkfbnvore3b52opmmi2ksk0.png)
=>
![[H_2O ] = 0.265 \ M](https://img.qammunity.org/2021/formulas/chemistry/college/vavebz6aan2jctior05qcpd9j7z3xwwd8h.png)
So
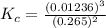
=>