Answer:
The partial pressure of carbon dioxide is 22.8 mmHg
Step-by-step explanation:
Dalton's Law is a gas law that relates the partial pressures of the gases in a mixture. This law says that the pressure of a gas mixture is equal to the sum of the partial pressures of all the gases present.
In this case:
Ptotal=Pnitrogen + Poxygen + Pcarbondioxide
You know that:
- Ptotal= 0.998 atm
- Pnitrogen= 0.770 atm
- Poxygen= 0.198 atm
- Pcarbondioxide= ?
Replacing:
0.998 atm=0.770 atm + 0.198 atm + Pcarbondioxide
Solving:
Pcarbondioxide= 0.998 atm - 0.770 atm - 0.198 atm
Pcarbondioxide= 0.03 atm
Now you apply the following rule of three: if 1 atm equals 760 mmHg, 0.03 atm how many mmHg equals?
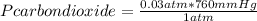
Pcarbondioxide= 22.8 mmHg
The partial pressure of carbon dioxide is 22.8 mmHg