Answer:
Extent of reaction = 95.9 mol.
Fractional conversion of the limiting reactant = 0.846.
Percentage by which the other reactant is in excess = 25.2 %.
Step-by-step explanation:
Hello.
In this case, for the undergoing chemical reaction:
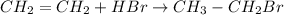
We can write the mole balance per species also including the extent of the reaction:
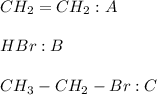
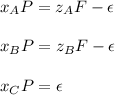
Considering that P is the flow of the outlet product. In such a way, writing the data we know, we can write:
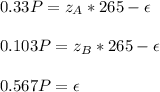
Whereas we can replace the C2H5Br mole balance in the others mole balances:
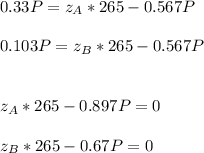
By knowing that
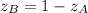 , we can write:
, we can write:
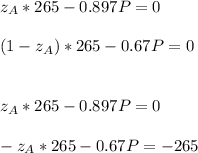
Thus, solving for P and
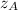 , we obtain:
, we obtain:
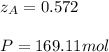
It means that the extent of the reaction is:
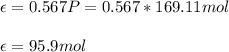
For the limiting reactant, due to the 1:1 mole ratio between the reactants, it is the one having the smallest flow rate:
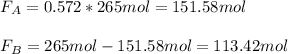
It means that the limiting reactant is B which is HBr, whose fractional conversion is:
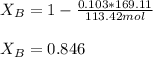
Finally, the percentage by which the other reactant is in excess, corresponds to:
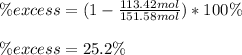
Regards.