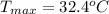Answer:
a
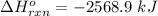
b
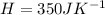
c
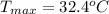
Step-by-step explanation:
From the question we are told that
The reaction of cyclobutane and oxygen is
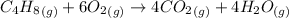
ΔH°f (kJ mol-1) : C4H8(g) = 27.7 ; CO2(g) = -393.5 ; H2O(g) = -241.8 ΔH° = kJ
Generally ΔH° for this reaction is mathematically represented as
![\Delta H^o _(rxn) = [[4 * \Delta H^o_f (CO_2_((g)) ) + 4 * \Delta H^o_f(H_2O_((g)) ] -[\Delta H^o_f (C_2H_6_((g)) + 6 * \Delta H^o_f (O_2_((g))) ] ]](https://img.qammunity.org/2021/formulas/chemistry/college/26auw0e93v5n4ajzp3bfbiffldh5izazw3.png)
=>
![\Delta H^o _(rxn) = [[4 * (-393.5) + 4 * (-241.8) ] -[ 27.7 + 6 * 0]](https://img.qammunity.org/2021/formulas/chemistry/college/hfhyxqygljcchr2l3n29ke8amcxz644m5l.png)
=>
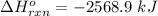
Generally the total heat capacity of 4 mol of CO2(g) and 4 mol of H2O(g), using CCO2(g) = 37.1 J K-1 mol-1 and CH2O(g) = 33.6 J K-1 mol-1. C = J K-1 is mathematically represented as
![H = [ 4 * C_{CO_2_((g))} + 6* C_{CH_2O_((g))}]](https://img.qammunity.org/2021/formulas/chemistry/college/qc3udj9y9onqbi2ftcgkqa3t4n1d5pgygh.png)
=>
![H = [ 4 * 37.1 + 6* 33.6 ]](https://img.qammunity.org/2021/formulas/chemistry/college/6qyr7pl8l02y09at9clb3v3g5bu7vzd4uw.png)
=>
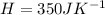
From the question the initial temperature of reactant is
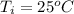
Generally the enthalpy change(
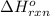 ) of the reaction is mathematically represented as
) of the reaction is mathematically represented as
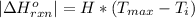
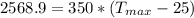
=>
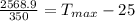
=>