Answer:
12 grams
Step-by-step explanation:
For any radioactive substance
the amount of substance left after n half life is given by =
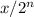
where x the original amount of substance.
____________________________________________
Half life of isotope Np-238 = 2 days
total no of days = 6
no of half life =total no of days/Half life of isotope Np-238 = 6/2 = 3
thus,
for our problem n = 3
initial amount of Np-238 = 96 grams
thus,
the amount of substance left after 3 half life = 96/2^3 = 96/8 = 12 grams.
Thus, 12 grams will be left after six days.