Given parameters:
Mass of H₃PO₄ = 49g
Unknown:
Number of moles = ?
Solution:
To find the number of moles of a given specie;
number of moles =

Molar mass = 3(1) + 31 + 4(16) = 98g/mol
Input the parameters and solve;
Number of moles =
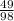 = 0.5mole
= 0.5mole
The number of moles is 0.5mole