Answer:
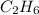
Step-by-step explanation:
Hello.
In this case, we can see that the mass of carbon of the unknown compound comes from the yielded mass of carbon dioxide, thus, we compute the moles of carbon as follows:
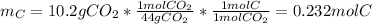
Moreover, the mass of hydrogen comes from the yielded water, therefore we can also compute the moles of water:
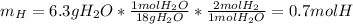
Then, to find the subscripts in the empirical formula, we divide by the moles of carbon as the smallest:
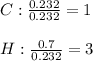
Whose molar mass is:
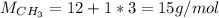
Thus, the ratio of the molecular formula to the empirical formula is:
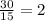
Therefore, the molecular formula is twice the empirical formula:
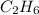
Which is actually ethane.
Regards.