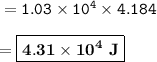The amount of energy in joules : 4.31 x 10⁴ J
Further explanation
Energy can be interpreted as the ability to do work
There are several examples of energy , including heat energy
The amount of heat is influenced by the mass of the object and the difference in temperature
Can be formulated
Q = m.c.ΔT
Q: heat received or removed by an object (J)
m: object mass (kg)
c: heat type substance (J / kg⁰C)
ΔT: temperature change (⁰C)
The amount of energy given off is 1.03x10⁴ cal.
Because 1 cal= 4.184 J, then