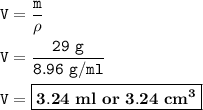
The volume of copper : 3.24 ml
Further explanation
Density is a quantity derived from the mass and volume
Density is the ratio of mass per unit volume
With the same mass, the volume of objects that have a high density will be smaller than objects with a smaller type of mass
The unit of density can be expressed in g/cm³ or kg/m³
Density formula:
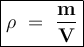
ρ = density , g/cm³ or kg/m³
m = mass , g or kg
v = volume , cm³ or m³
A common example is the water density of 1 gr / cm³
The density of copper : 8.96 gr/ml
mass of copper : 29 g
then the volume :