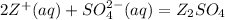Answer:
Step-by-step explanation:
The reaction between Z(aq) and Na2SO4 forming a brown ppt shows that the product formed from this reaction is a solid. and also it can be seen that Z+ is a monocationic while SO4 is dianionic, hence molecules (2) of Z+ will combine with (1) molecule of SO4
below is the balanced chemical equation for this reaction