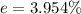Answer:
1
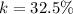
2
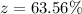
3
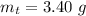
4
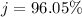
5
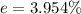
Step-by-step explanation:
From the question we are told that
The mass of the sample is
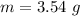
The mass of the AgNO3 is
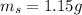
The mass of Mg(OH)2 is
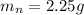
Generally the percentage of AgNO3 in the mixture is
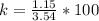
=>
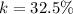
Generally the percentage of Mg(OH)2 in the mixture is
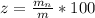
=>
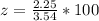
=>
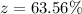
Generally the total mass of the AgNO3 and Mg(OH)2 recovered is
![[tex]m_t = m_s +m_n](https://img.qammunity.org/2021/formulas/chemistry/college/tgunahzruvmlg1zmuainx1t3kj0dkde3pj.png)
=>
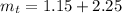
=>
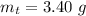
Generally the percentage recovery of the components is mathematically represented as
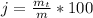
=>
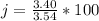
=>
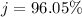
Generally the percentage error for the separation of the components of the mixture is mathematically represented as
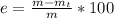
=>
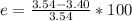
=>