Answer:
a. c= silver/ 234J(kg/K) > b. c= water/ 4190 J(kg/K) > c. c= glass/ 754J/(kg.K)
Step-by-step explanation:
Hello.
In this case, since the heat resulting from the temperature change for an specified amount of a substance is defined via:
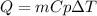
Since the specific heat is related to amount of energy required to raise the temperature of 1 kg of a substance by 1 °C, we can infer that the higher the specific heat, the higher the required energy as they are in a directly proportional relationship. Moreover, since the specific heat and the change in temperature are in an inversely proportional relationship, we can infer that the higher the specific heat, the lower the temperature change, therefore, we can rank the substances follows:
a. c= silver/ 234J(kg/K) > b. c= water/ 4190 J(kg/K) > c. c= glass/ 754J/(kg.K)
It means that silver will produce the largest temperature change, next water and finally glass since silver has the smallest specific heat next water and finally glass.
Best regards.