Answer:
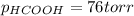
Step-by-step explanation:
Hello.
In this case, since the total pressure at any point of the experiment is written via the Dalton's law:
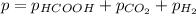
We can also write the partial pressure of formic acid in terms of its initial pressure (220 torr) and the change
 as the time goes by:
as the time goes by:
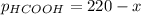
Thus, based on the stoichiometry, since it is a first-order decay and all the stoichiometric coefficients are 1, we can infer that the decrease in the partial pressure of formic acid equals the increase in the partial pressure of both carbon dioxide and hydrogen, therefore we can write:
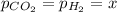
In such a way, we write the Dalton's law as shown below:
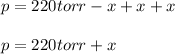
Thus, at the point in which the total pressure is 364 torr, the change is:

It means, that the partial pressure of formic acid is:
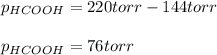
Best regards.