Answer:
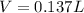
Step-by-step explanation:
Hello.
In this case, since the molarity is computed in terms of the moles of solute (acetic acid) and the volume of the solution in liters, we must first compute the moles of acetic acid by using its molar mass to subsequently compute the volume as follows:
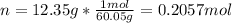
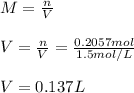
Best regards.