A. The rate of reaction A is lower
Further explanation
The reaction rate (v) shows the change in the concentration (reduction of reactants and product additions ) per unit time
Can be formulated:
Reaction: aA ⇒ bB
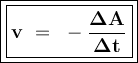
or
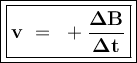
A = reactants
B = product
v = reaction rate
t = reaction time
Time of reaction A : 12 s
Time of reaction B : 2 s
Because reaction speed/the rate of reaction is inversely proportional to the time, then A. The rate of reaction A is lower