Answer:
Lower than it should be.
Step-by-step explanation:
Hello.
In this case, since the density is computed by:
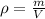
And we obtain the volume of the solid by substracting the mass of the water and the solid minus the mass of water:
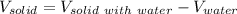
If some water is splashed out of the cylinder, the volume of water will be lower than originally measured, it means that the volume of the solid will be higher than real. In such a way, since the density is in an inversely proportional relationship with volume, as the volume of the solid is wrongly increased, therefore the density of the solid will be lower than in should be.
Regards.