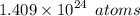
Step-by-step explanation:
Convert from moles to atoms:
2.34 mol titanium (Ti)
Determine the amount of substance, n:
n = 2.34 mol
Look up Avogadro's constant,
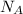 , to find the number of atoms in a mole:
, to find the number of atoms in a mole:
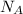 = 6.022×10^23 atoms/mol
= 6.022×10^23 atoms/mol
Multiply n by
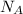 to convert to the number of atoms,
to convert to the number of atoms,
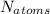 :
:
Precise Answer:
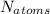 = n×
= n×
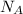 = (2.34 mol)/1×(6.022×10^23 atoms)/(1 mol) = 1.409×10^24 atoms
= (2.34 mol)/1×(6.022×10^23 atoms)/(1 mol) = 1.409×10^24 atoms