Answer:
1) 13 mg/liters
2) 72.22 mg/lit
3) The Alkanity in raw water is not sufficient enough and the kinds of chemicals needed to enhance its acidity are
- CaO
- KOH
- Na2CO3
- NaOH
- CO2
- NaHCO3
Step-by-step explanation:
1) plot of turbidity versus dose and optimal dose of Alum ( mg/L )
Optimal dose of Alum = 13 mg/liters from the graph attached below
2) Theoretical amount of alkalinity consumed at the optimal dose can be calculated as follows
Alkanity is due to
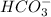
given optimal dose of Alum = 13 mg/liters for question 1
I mole of alum = 2 moles of AL(OH)3
666 grams of alum = 2*27 = 54 grams of AL(OH)3
hence 1 mole of
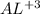 = (13/54 ) mMole / lit
= (13/54 ) mMole / lit
The moles of HCO3 = 6 *
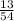 because 1 mole of Alum reacts with 6 moles of HC03
because 1 mole of Alum reacts with 6 moles of HC03
[HCO3] as CaCO3 = 6 * (13/54) * 50
= 72.22 mg/lit (theoretical amount of alkalinity consumed)
3) The Alkanity in raw water is not sufficient enough and the kinds of chemicals needed to enhance its acidity are
- CaO
- KOH
- Na2CO3
- NaOH
- CO2
- NaHCO3