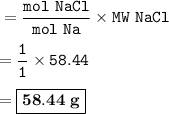Answer : 58.44 g
Further explanation
The reaction
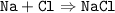
mole ratio :
Na : Cl : NaCl = 1 : 1 : 1
Molar mass of Na : 22,989769 g/mol = 22.99 g/mol
Molar mass of Cl : 35,453 g/mol= 35.45 g/mol
Molar mass of NaCl : 58,44 g/mol
Let's check the mol for reactant (Na and Cl) and product(NaCl)
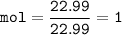
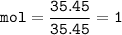
Then the reactants are completed reacting because it matches the mole ratio, which is 1: 1
so that the mole of the product can be determined from the mol Na or Cl, which is 1