Answer:

Step-by-step explanation:
In the first option, the compound
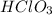 has 1 hydrogen atom and 3 Oxygen atoms. There are 3 times as many oxygen atoms as hydrogen atoms.
has 1 hydrogen atom and 3 Oxygen atoms. There are 3 times as many oxygen atoms as hydrogen atoms.
In the second option, the compound
 has 2 hydrogen atom and 4 Oxygen atoms. That is 2:4 = 1:2. Therefore, there are twice as many oxygen atoms as hydrogen atoms.
has 2 hydrogen atom and 4 Oxygen atoms. That is 2:4 = 1:2. Therefore, there are twice as many oxygen atoms as hydrogen atoms.
In the third option, the compound
 has 2 hydrogen atoms and 1 Oxygen atom. There are ½ times as many oxygen atoms as hydrogen atoms.
has 2 hydrogen atoms and 1 Oxygen atom. There are ½ times as many oxygen atoms as hydrogen atoms.
In the fourth option, the compound
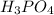 has 3 hydrogen atoms and 4 Oxygen atoms. 4 oxygen atoms to 3 hydrogen atoms.
has 3 hydrogen atoms and 4 Oxygen atoms. 4 oxygen atoms to 3 hydrogen atoms.