Answer:
The total amount of heat is 250.64 kcal.
Step-by-step explanation:
Given that,
Total amount of heat = 2.000 kg
Temperature of snow = -15.00°C
Temperature of water = 38.00°C
Heat of fusion of water = 1.44 kcal/mol
Specific heat of solid water = 0.488 cal/g°C
Specific heat of liquid = 1.00 cal/g°C
We need to calculate the heat required to bring temperature -15°C to 0°C snow
Using formula of heat
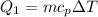
Put the value into the formula
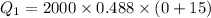
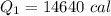
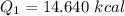
We need to calculate the heat required to convert 0°C snow into 0°C water
Using formula of heat
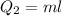
Put the value into the formula
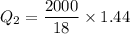
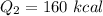
We need to calculate heat required to increases temperature of water from 0°C to 38°C
Using formula of heat
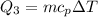
Put the value into the formula
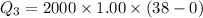
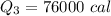
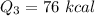
We need to calculate the total heat
Using all heat amount
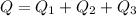
Put the value into the formula
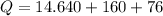
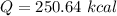
Hence, The total amount of heat is 250.64 kcal.